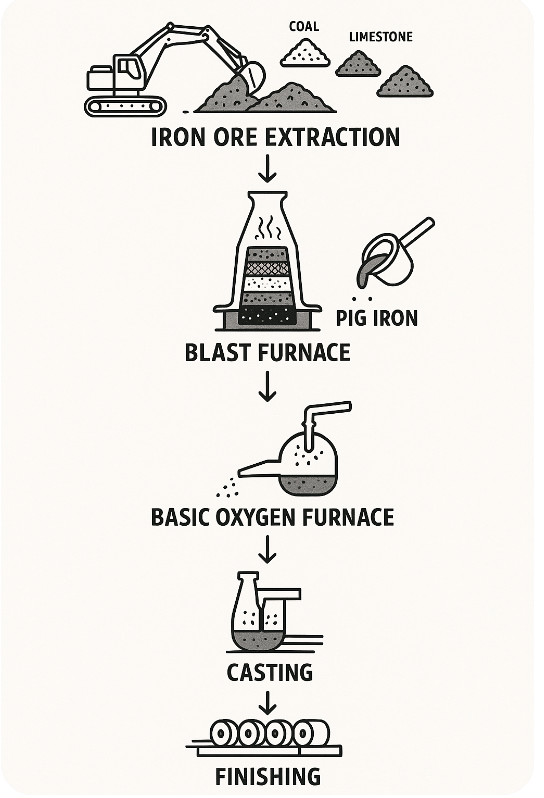
Basic steel manufacture
Traditional Steelmaking Processes
Steel production transforms iron ore through three essential stages: reduction, melting, and refining. During reduction, iron compounds in ore undergo chemical reactions with carbon or hydrogen at high temperatures to convert into metallic iron. The melting stage heats all metallic iron and impurities until complete liquefaction occurs, allowing impurities that resist reduction (primarily silica and alumina, termed gangue) to form molten slag that floats on molten iron for easy separation. Refining removes excess dissolved elements including carbon, phosphorus, and sulfur from molten iron to meet steel specifications.
Different production routes handle these stages in varying configurations. The blast furnace route achieves both reduction and melting within the furnace itself, with refining performed separately in a Basic Oxygen Furnace. Electric arc furnace and electric smelting furnace routes utilize DRI units that perform only reduction without melting, requiring subsequent melting and refining to remove impurities remaining in solid DRI.
Raw Materials and Preparation
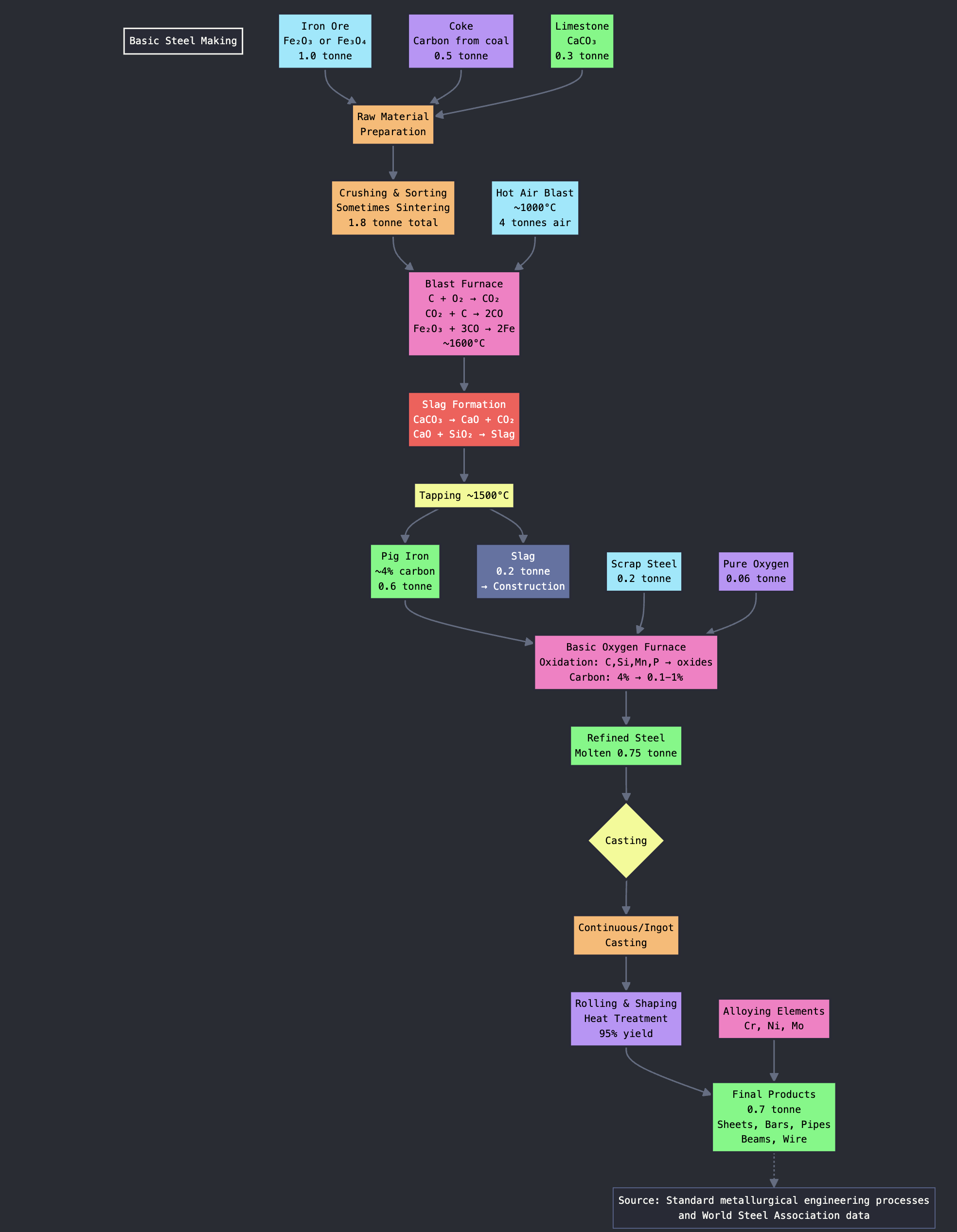
Steel production begins with careful preparation of essential raw materials. Iron ore, typically hematite (Fe₂O₃) or magnetite (Fe₃O₄), provides the iron source. Coke, a carbon form derived from coal, serves dual functions as fuel and reducing agent. Limestone (calcium carbonate, CaCO₃) acts as flux to remove impurities during processing.
These materials undergo crushing, sorting, and sometimes sintering processes where partial fusion creates lumps that improve blast furnace efficiency. Proper preparation ensures optimal chemical reactions and material flow through subsequent processing stages.
Blast Furnace Operations
The blast furnace represents a sophisticated metallurgical reactor consisting of a tall steel structure lined with refractory brick. Operations begin with loading alternating layers of iron ore, coke, and limestone from the top. Hot air, preheated to approximately 1000°C, is injected through bottom nozzles called tuyeres, creating the characteristic blast that gives the furnace its name.
Critical chemical reactions occur throughout the furnace height. Combustion reactions convert coke and oxygen to carbon dioxide (C + O₂ → CO₂), while subsequent reduction reactions produce carbon monoxide (CO₂ + C → 2CO) that reduces iron ore to metallic iron (Fe₂O₃ + 3CO → 2Fe + 3CO₂). Simultaneously, limestone decomposes to calcium oxide (CaCO₃ → CaO + CO₂), which reacts with silica and other impurities to form slag that floats above molten iron.
The furnace operates continuously with periodic tapping to drain pig iron from the bottom at approximately 1500°C, while slag is removed separately for use in construction materials and other applications.
Basic Oxygen Furnace Steelmaking
Pig iron from blast furnaces contains approximately 4% carbon, requiring refining to achieve steel specifications. The Basic Oxygen Furnace receives molten pig iron along with scrap steel additions that provide cooling and recycling benefits. High-pressure pure oxygen streams are blown into the molten iron, creating vigorous reactions.
Oxygen reacts with carbon, silicon, manganese, and phosphorus to form oxides that either burn off as gases (CO, CO₂) or are captured in slag. This process reduces carbon content to 0.1-1% depending on desired steel grade specifications. Temperature and chemical composition are carefully monitored throughout the refining cycle to ensure product quality.
Casting and Finishing Operations
Purified molten steel undergoes casting through two primary methods. Ingot casting involves pouring steel into large molds for solidification, while continuous casting flows steel into cooled molds where it solidifies while being drawn downward into slabs, billets, or blooms. Continuous casting offers superior efficiency and product consistency.
Semi-finished steel forms undergo extensive finishing operations including rolling, shaping, and heat treatment to produce final products. These processes create diverse steel products including sheets, bars, pipes, structural beams, and wire. Specialty steel production incorporates additional alloying elements such as chromium, nickel, and molybdenum to achieve specific properties required for applications like stainless steel manufacturing.
The complete steel production chain demonstrates the sophisticated transformation of raw iron ore into engineered materials that form the foundation of modern industrial infrastructure and consumer products.